 The
Therapeutics Initiative's objectives are unbiased review
and dissemination of therapeutic evidence. Our
recommendations are intended to apply to most patients;
exceptional patients require exceptional approaches. We
are committed to evaluate the effectiveness of our
educational activities using the Pharmacare/PharmaNet
databases without identifying individual physicians,
pharmacies or patients. The Therapeutics Initiative is
funded by the BC Ministry of Health through a 5-year
grant to the University of BC. The Therapeutics
Initiative provides evidence based advice about drug
therapy, and is not responsible for formulating or
adjudicating provincial drug policies. The
Therapeutics Initiative's objectives are unbiased review
and dissemination of therapeutic evidence. Our
recommendations are intended to apply to most patients;
exceptional patients require exceptional approaches. We
are committed to evaluate the effectiveness of our
educational activities using the Pharmacare/PharmaNet
databases without identifying individual physicians,
pharmacies or patients. The Therapeutics Initiative is
funded by the BC Ministry of Health through a 5-year
grant to the University of BC. The Therapeutics
Initiative provides evidence based advice about drug
therapy, and is not responsible for formulating or
adjudicating provincial drug policies. |
Click
here
to download a printable version of this
Therapeutics Letter in Adobe Acrobat PDF format (63 KB).
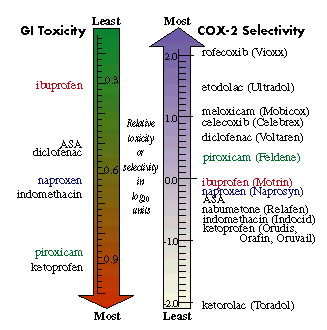
Figure: Does COX-2
selectivity explain GI toxicity?
Selective
COX-2 inhibitors: Are they safer NSAIDs?
This Therapeutics Letter adds to information about NSAIDs in Letter
4 and Letter 17. It also responds to our
commitment in Letter 31 to report on celecoxib when
published trials became available.
What is the baseline risk of peptic ulcer
complications?
The risk of hospitalization due to peptic ulcer
complication is about 0.2% per year in non-users of NSAIDs (Saskatchewan data
from 1982-1986).1 The most common presentation is
gastrointestinal (GI) hemorrhage. Risk is higher in men than women and increases
with age.1
What is the risk of peptic ulcer complications in
patients taking NSAIDs?
Using data based on community dispensed NSAIDs, baseline
incidence is increased 4-fold in patients currently taking NSAIDs.1,
2 The risk varies widely with different NSAIDs3
and is illustrated in the left arrow of the Figure.
Risk also varies with dose, 2.5 fold for low doses and 8.5
fold for high doses.3 Ibuprofen has
the lowest risk, 2.0 fold, estimated absolute risk increase (ARI) 0.2%, number
needed to harm (NNH) 500 per year.2
What can be done to reduce the risk of NSAID GI
toxicity?
-
prescribe NSAIDs only to patients who do not respond
to acetaminophen
-
select the NSAID with the lowest GI toxicity
-
prescribe the lowest possible dose for the shortest
duration of time2
Why might selective COX-2 inhibitors cause less peptic
ulcer complications?
Potential for reduced GI toxicity with selective COX-2
inhibitors is based on the hypothesis that inhibition of COX-1 by effects on GI
mucosa and platelets is the main cause of GI ulcers and bleeding. Testing by a
single laboratory using human assay systems shows that rofecoxib is the most
selective of available NSAIDs (>50-fold potency for COX-2 over COX-1).4
The relative COX-2 selectivity of common NSAIDs are shown in the right hand
arrow of the Figure. The order of COX-2 selectivity
does not appear to explain the order of GI toxicity.
How is the GI toxicity of different NSAIDs best
compared?
Comparing different NSAIDs should be done in head-to-head
randomized controlled trials (RCTs) using minimum effective doses and measuring
serious GI outcomes. The most critical outcome to the patient is peptic ulcer
complication (hemorrhage, perforation or obstruction) leading to
hospitalization. A less serious but important outcome is symptomatic peptic
ulcer (possibly requiring outpatient investigation and 6-8 weeks of treatment).
Because these 2 outcomes have substantially different implications to the
patient, they should be reported separately.
Do the newer more selective COX-2 inhibitors cause
less serious GI toxicity?
Four published head-to-head RCTs provide a partial answer
to this question. Unfortunately, in all these trials a single dose of drug was
administered to all patients and not in a manner to minimize toxicity i.e.
titrating the dose to the minimum effective dose. The CLASS trial5
randomized 7968 patients to 3 groups, celecoxib 400 mg BID, ibuprofen 800 mg TID
and diclofenac, 75 mg BID; patients (69% female, mean age 60) with
osteoarthritis (72.6%) and rheumatoid arthritis were treated for an average
duration of 4.2 months.5 In the VIGOR trial6,
rofecoxib 50 mg per day was compared to naproxen 500 mg BID in 8076 patients
(80% female, mean age 58) with rheumatoid arthritis over a median treatment
period of 9 months.6 The Table
shows the major outcomes from these 2 trials. Only rofecoxib significantly
reduced complicated ulcers. While subgroup analyses were reported in these
trials, these should be used to design future trials, not to support conclusions
or clinical decisions.
Two large short-term RCTs (28 days) compared meloxicam with
other NSAIDs in osteoarthritis patients. MELISSA7
(9323 patients) compared meloxicam 7.5 mg daily with diclofenac SR 100 mg daily
and SELECT8 (8656 patients) compared meloxicam
7.5 mg daily with piroxicam 20 mg daily. Interpretation of these trials is
difficult as the doses were not equi-effective; measures of efficacy in the
meloxicam patients were significantly less than the comparator in both trials.
Complicated and symptomatic ulcers were uncommon and not significantly
different. Total withdrawals due to adverse events were lower, 6.0%, with
meloxicam than diclofenac, 8.0%, or piroxicam, 7.2%.7, 8
Are the newer selective COX-2 inhibitors safer
overall?
The best measure of overall safety, serious adverse events,
is a required outcome of all clinical trials. This category includes death,
life-threatening events, events leading to or prolonging hospitalization, and
cancers. Total serious adverse events were not lower with the new drugs:
celecoxib (see Table);
meloxicam, 1.2%, versus diclofenac,
1.1%;7 and meloxicam,
0.6%, versus piroxicam, 0.7%.8 This outcome
was not reported in the VIGOR paper, however, total withdrawals due to adverse
events were similar for rofecoxib and naproxen, and myocardial infarctions were
significantly increased in the rofecoxib group (see Table).6
Withdrawals due to rash were significantly increased in the celecoxib group,
2.7% vs ibuprofen/diclofenac, 1.2%, ARR = 1.5%, NNH = 67.5
Withdrawals due to renal adverse events were similar for the new drugs and
the comparators.
What important data is not provided in these RCT
publications?
CLASS: death and serious adverse events by category and
cause, separate complete data for ibuprofen and diclofenac, incidence of GI
investigations in 3 treatment groups.5
VIGOR: serious adverse events and withdrawals due to
adverse events by category and
cause, incidence of GI investigations.6
MELISSA and SELECT: death, serious adverse events and
withdrawals due to adverse events by category and cause.7,
8
What is the dose and cost of newer NSAIDs?
Celecoxib 100 to 200 mg BID, cost $1.37-2.71 daily.
Meloxicam 7.5 to 15 mg daily, cost $0.83–0.99 daily. Rofecoxib 12.5 to
25 mg daily, cost $1.34 daily (for either dose). See Therapeutics
Letter 4 for dose and cost of other NSAIDs. The costs have been updated to 2000 prices on our website.
Conclusions
-
Patients on rofecoxib had less complicated and
symptomatic ulcers but more myocardial infarctions than patients on
naproxen.
-
COX-2 selective NSAIDs were associated with
the same incidence of serious adverse events as
non-selective NSAIDs.
-
Celecoxib
and meloxicam caused fewer withdrawals due to adverse events than
non-selective NSAIDs.
-
Head-to-head RCTs comparing NSAIDS with dosing
regimens to optimize efficacy and safety would be useful.
Table: Major outcomes in order of most to least serious
from CLASS5 and VIGOR6
trials
|
Outcome |
Celecoxib
% |
Other*
% |
RR
95%CI |
ARR
% |
NNT
4 mo |
Rofecoxib
% |
Naproxen
% |
RR
95%CI |
ARR
ARI
% |
NNT
NNH
9 mo |
Myocardial
infarction |
0.3 |
0.3 |
0.9
0.4-2.1 |
NS |
NS |
0.4 |
0.1 |
4.0
1.3-12 |
0.3 |
333 |
Complicated
ulcers |
0.3 |
0.6 |
0.6
0.3-1.2 |
NS |
NS |
0.4 |
0.9 |
0.4
0.2-0.8 |
0.5 |
200 |
Serious
adverse events |
4.3 |
4.2 |
1.02
0.8-1.3 |
NS |
NS |
NR |
NR |
|
|
|
Symptomatic
ulcers |
0.5 |
0.7 |
0.65
0.4-1.2 |
NS |
NS |
1.0 |
2.1 |
0.5
0.3-0.7 |
1.1 |
91 |
Withdrawals due
to adverse events |
18.4 |
20.6 |
0.89
0.8-0.97 |
2.2 |
44 |
16.4 |
16.1 |
1.02
0.9-1.1 |
NS |
NS |
* Half ibuprofen and half diclofenac
RR=Risk Reduction
CI=confidence Interval
ARR=Absolute Risk Reduction
NNT=Number Needed to Treat to prevent one event
ARI=Absolute Risk Increase
NNH=Number Needed to cause one Harmful event
NS=Non-Significant
NR=Not Reported
|
This
Therapeutics Letter contains an assessment and synthesis
of published (and whenever possible peer-reviewed)
publications up to December 2000. We attempt to
maintain the accuracy of the information contained in the
Therapeutics Letter by extensive literature searches and
verification by both the authors and the editorial board.
In addition this Therapeutics Letter was submitted for
review to 120 experts and primary care
physicians in order to correct any identified
shortcomings or inaccuracies and to ensure that the
information is concise and relevant to clinicians.
|
References
-
García Rodriguez LA, Walker AM, Pérez
Gutthann, S. Nonsteroidal
antiinflammatory drugs and gastrointestinal hospitalizations in
Saskatchewan: a cohort study. Epidemiology. 1992; 3:337-342.
-
Langman MJS, Weil J, Wainwright P, et
al. Risks of bleeding peptic ulcer associated with individual
non-steroidal anti-inflammatory drugs. Lancet. 1994; 343:1075-78.
-
Henry D, Lim LL-Y, Garcia Rodriguez LA,
et al. Variability in risk of gastrointestinal complications with
individual non-steroidal anti-inflammatory drugs: results of a collaborative
meta-analysis. BMJ. 1996; 312:1563-6.
-
Warner TD, Giuliano F, Vojnovic I, et
al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than
cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: A
full in vitro analysis. Proc Natl Acad Sci USA. Pharmacology. 1999;
96:7563-7568.
-
Silverstein FE, Faich G, Goldstein JL,
et al. Gastrointestinal toxicity with celecoxib versus nonsteroidal
anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. The
CLASS study: a randomized controlled trial. JAMA. 2000; 284:1247-1255.
-
Bombardier C, Laine L, Reicin A, et al,
for the VIGOR Study Group. Comparison of upper
gastrointestinal toxicity of rofecoxib and naproxen in patients with
rheumatoid arthritis. N Engl J Med. 2000; 343:1520-8.
-
Hawkey C, Kahan A, Steinbrück K, et al.
Gastrointestinal tolerability of meloxicam compared to
diclofenac in osteoarthritis patients. Br. J. Rheumatol. 1998;
37:937-945.
-
Dequeker J, Hawkey C, Kahan A, et al. Improvement
in gastrointestinal tolerability of the selective cyclooxygenase
(COX)-2 inhibitor, meloxicam, compared with piroxicam: results of the safety
and efficacy large-scale evaluation of COX-inhibiting therapies (SELECT)
trial in osteoarthritis. Br. J. Rheumatol.1998; 37:946-951.
Please send feedback!
Back to Therapeutics Letter.
 Back to
the Therapeutics Initiative Home Page.
Back to
the Therapeutics Initiative Home Page.
©Therapeutics Initiative. Last updated: February 6, 2001.
 The
Therapeutics Initiative's objectives are unbiased review
and dissemination of therapeutic evidence. Our
recommendations are intended to apply to most patients;
exceptional patients require exceptional approaches. We
are committed to evaluate the effectiveness of our
educational activities using the Pharmacare/PharmaNet
databases without identifying individual physicians,
pharmacies or patients. The Therapeutics Initiative is
funded by the BC Ministry of Health through a 5-year
grant to the University of BC. The Therapeutics
Initiative provides evidence based advice about drug
therapy, and is not responsible for formulating or
adjudicating provincial drug policies.
The
Therapeutics Initiative's objectives are unbiased review
and dissemination of therapeutic evidence. Our
recommendations are intended to apply to most patients;
exceptional patients require exceptional approaches. We
are committed to evaluate the effectiveness of our
educational activities using the Pharmacare/PharmaNet
databases without identifying individual physicians,
pharmacies or patients. The Therapeutics Initiative is
funded by the BC Ministry of Health through a 5-year
grant to the University of BC. The Therapeutics
Initiative provides evidence based advice about drug
therapy, and is not responsible for formulating or
adjudicating provincial drug policies. 